📢 Attention all medical writers, publication professionals and clinical researchers! 📢
You have been waiting for 15 years, and now it’s there! 🏆
Yes, you have guessed right – we are talking about the CONSORT 2025 statement simultaneously published yesterday in The BMJ, JAMA, The Lancet, Nature Medicine and PLOS Medicine.
⭐ The Consolidated Standards of Reporting Trials (CONSORT) initiative aimed to improve and standardise the reporting of clinical trials in medical and scientific literature. Specifically, the CONSORT checklist, established in the 90s, and last updated in 2010, covered the publications of randomised controlled trials. ⭐
For many years, the CONSORT checklist and the SPIRIT checklist that covers publication of trial protocols, guided the development of clinical trial-related publications:
✅ Many journals include the CONSORT checklist in their submission guidelines
✅ CONSORT and SPIRIT checklists are widely used by authors and medical writing professionals as a framework for publication writing
✅ CONSORT and SPIRIT checklists have informed aspects of clinical trial design
❗ Both checklists have now been updated: see a summary of key updates below and check out the original publications. 🏆
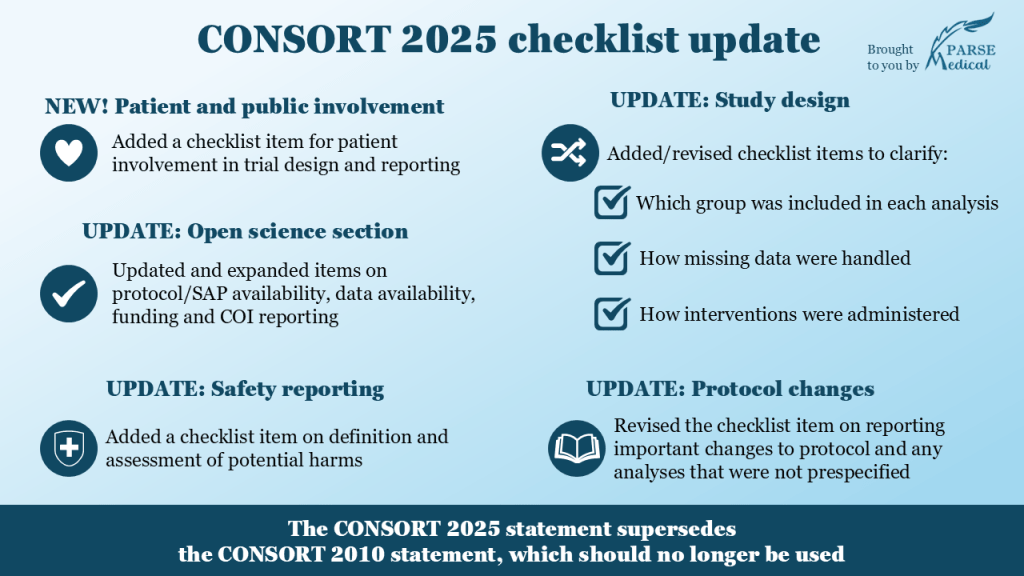
✅ One notable change is the added checklist item 8 asking to provide details of patient and public involvement in the design, conduct and reporting of the trial. ⭐ The CONSORT-SPIRIT working group is also working on a dedicated website that will contain additional resources and training materials for patient and general public (and, of course, researchers, journal editors and peer reviewers). ⭐
❗ Not covered here, but also important, are the changes to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement and checklist. One important update to highlight is the requirement to justify the use of surrogate endpoints:
✅ If a surrogate is used for the primary endpoint, the authors are required to highlight it, include the target outcome that has been substituted for the surrogate, and justify the use of surrogate by providing validation evidence and its impact on estimating the sample size.
✅ Importantly, the authors should clarify if the trial participants were informed that the trial was based on a surrogate endpoint.
To learn more about the updates, please refer to the original publications:
- Hopewell S, et al. JAMA. Published online April 14, 2025. doi:10.1001/jama.2025.4347 (https://jamanetwork.com/journals/jama/fullarticle/2832868)
- Chan AW, et al. JAMA. Published online April 14, 2025. doi:10.1001/jama.2025.4486 (https://jamanetwork.com/journals/jama/fullarticle/2833408)
- The new joint SPIRIT-CONSORT website https://www.consort-spirit.org (available soon)

Leave a comment